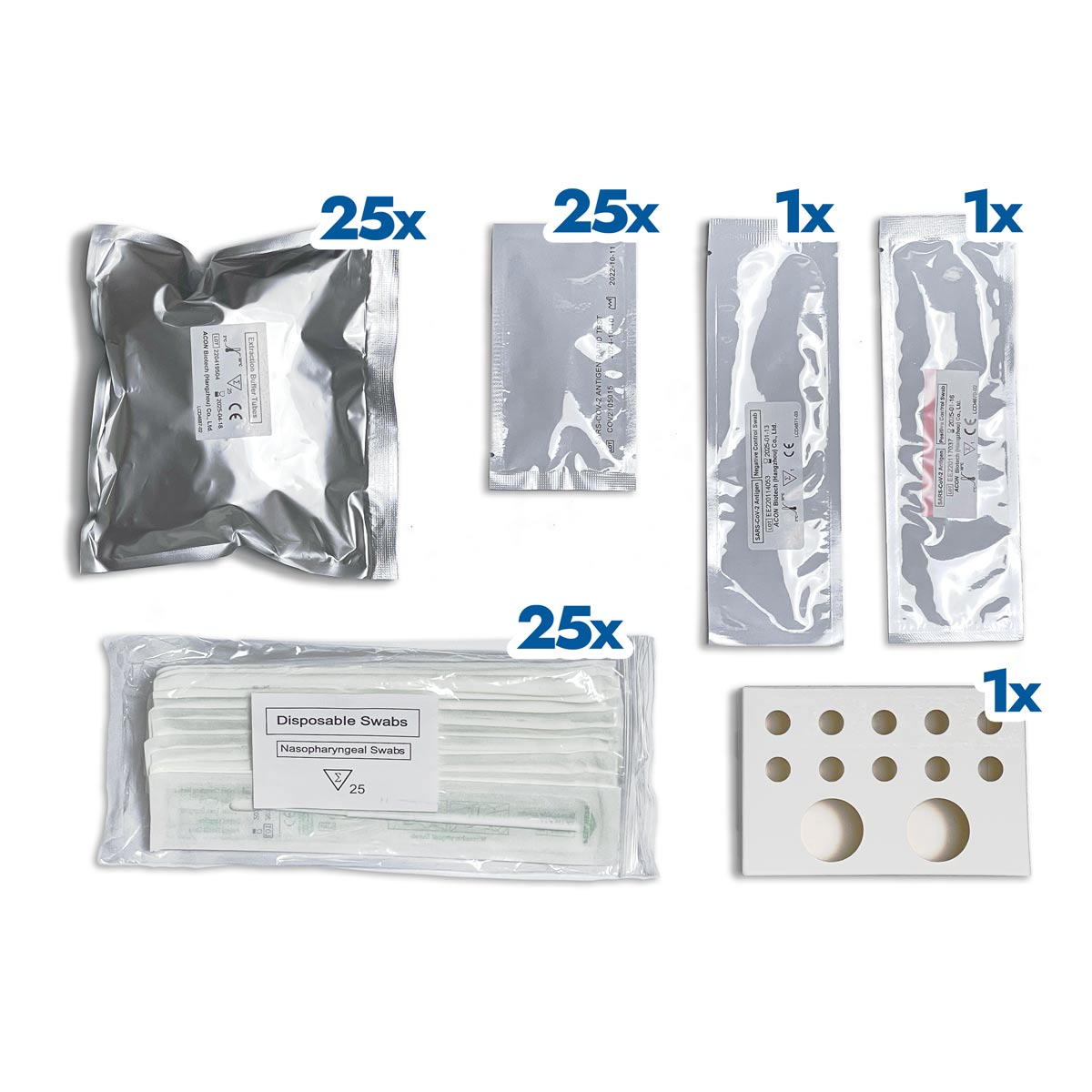
Flowflex rapid antigenic test for professional use
Intended Use: The Rapid Antigen Test for the qualitative detection of nucleocapsid antigens of SARS-CoV-2 in nasal and nasopharyngeal swab samples. For professional in vitro diagnostic use only.
Buy
Intended use:
The rapid antigen test for SARS-CoV-2 is a lateral flow immunochromatographic assay for the qualitative detection of SARS-CoV-2 nucleocapsid antigen in nasal and nasopharyngeal swab samples taken from individuals deemed potentially infected with COVID-19 by their physicians within the first seven days of symptom manifestation. The rapid antigen test for SARSCoV-2 can be used to test samples from asymptomatic subjects. The rapid antigen test for SARS-CoV-2 does not distinguish between SARS-CoV and SARS-CoV-2.
The rapid antigen test for SARS-CoV-2 is intended for use by trained clinical laboratory personnel and trained health care workers at care centers.
Summary:
The new coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. Humans are generally susceptible to such infection. Currently, patients infected with the new coronavirus are the main source of infection, but asymptomatic infected persons may also be a source of infection. Based on the current epidemiological survey, the incubation period is between 1 and 14 days, mainly between 3 and 7 days. The main manifestations are fever, fatigue and dry cough, loss of sense of smell, and respiratory failure. In some cases, nasal congestion, rhinorrhea, sore throat, myalgia, and diarrhea are present.
Buy
Principle:
When the extracted samples are processed and dispensed into the test cassette, SARS-CoV-2 antigens, if present in the sample, react with particles coated with antiSARS-CoV-2 antibodies, which are present on the test line (T ). The mixture then migrates across the membrane by capillary action. Antigen-conjugate complexes migrate across the test strip to the reaction area and are captured by a line of membrane-bound antibodies. Test results are visually interpreted after 15-30 minutes based on the presence or absence of visible colored lines. As an exclusive procedural control, a colored line is always displayed in the control line region (C ) indicating that the correct volume of sample has been added and that correct membrane imbibition has occurred.
Reagents:
The test cassette contains anti-SARS-CoV-2 antibodies. The positive control swab contains recombinant SARS-CoV-2 antigen pre-coated on the swab.
Precautions:
- For professional in vitro diagnostic use only. Do not use beyond the expiration date.
- Do not eat, drink or smoke in the area where samples or the kit are handled.
- Do not use the test if the outer casing is damaged.
- Handle all samples as if they contained infectious agents. Observe established precautions against biohazards during testing and follow standard procedures for proper disposal of samples.
- Wear protective clothing, such as lab coats, disposable gloves, masks and eye protection when samples are tested.
- The test used must be disposed of in accordance with local regulations. The test used should be considered potentially infected and disposed of in accordance with local regulations.
- Humidity and temperature can adversely affect the results.
- Read the package insert carefully before performing the test. Failure to follow the instructions specified in the package insert may result in inaccurate test results.
- The test line for a sample with a high viral load may become visible within 15 minutes, or as soon as the sample crosses the area of the test line.
- The test line for a low viral load sample becomes visible after 30 minutes.
Preservation and stability:
- The kit can be stored at temperatures between 2 – 30 °C.
- The test is stable until the expiration date printed on the sealed outer wrapper.
- The test should remain in the sealed wrapper until use.
- DO NOT FREEZE.
- Do not use beyond the expiration date.
Limitations:
- The rapid antigen test for SARS-CoV-2 is intended for in vitro diagnostic use only. The test should only be used to detect SARS-CoV-2 antigens in nasal and nasopharyngeal swab samples. The intensity of the test line does not necessarily correlate with the SARS-CoV-2 viral titer present in the sample.
- Samples should be tested as quickly as possible after the sample is taken and no later than within an hour after the sample is taken.
- The use of storage and stabilization media (media) to transport the sample may result in reduced sensitivity to the test.
- A false-negative test could be obtained if the antigen concentration in a sample was below the detection limit of the test or if the sample was collected incorrectly.
- Test results should be correlated with other clinical data available to the physician.
- A positive test result does not rule out co-infection with other pathogens.
- A positive test result does not distinguish between SARS-CoV and SARS-CoV-2.
- A negative test result is not intended to rule out other viral or bacterial infections.
- A negative result, on a patient with the onset of symptoms beyond seven days, should be treated as presumptive and confirmed by molecular testing, based on which clinical treatment should be determined.
(If differentiation of specific SARS viruses and strains is needed, further testing should be done.)